As the Density of a Substance Increases the Volume
The density calculator supports metrics in both metric and imperial units. The density of solids and liquids normally increase with decreasing temperature.

Temperature And Density Chapter 3 Density Middle School Chemistry
Kg pounds tons tonnes cu ft cu yd mm3 cm3 m3 and so on.

. Water has a density of 1 kilogram per liter while air has. In practical terms density is the weight of a substance for a specific volume. When salt is dissolved in fresh water the density of the water increases.
If volume increases without an increase in mass then the density decreases Fig. If the volume remains the same and the mass increases then the density must also increase. Gm 3Dry air mostly consists of nitrogen 78 and oxygen 21 The remaining 1 contains many different gases among others argon carbon dioxide neon or heliumHowever the air will cease to be dry air when water vapor appears.
Density is the amount of matter in an object compared to its volume the amount of space it takes up. Ice is less dense than liquid water which is why your ice cubes float in your glass. The standard unit of specific volume is cubic meters per kilogram m 3 kg but other units include ft 3 lb ft.
But water contracts when cooled up to 40C but expands when cooled further below 40C. The calculator can also be used to solve for mass or volume given density and the other of the two. Why does density depend on.
Density is a physical parameter that plays a vital and important role in all material states whether solid liquid or gaseous. As you might expect water density is an important water measurement. It is also a convenient property because it provides a link or conversion factor between the mass and the volume of a substance.
Cold water is more dense and will sink in room-temperature water. The density of water is 1 gram per cubic centimeter. Mathematically density is defined as mass divided by volume.
The density of air is usually denoted by the Greek letter rho or ρ and it measures the mass of air per unit volume eg. Why does density change when temperature changes. In thermodynamics the specific volume of a substance symbol.
In contrast to these increases several pathological conditions eg major depression Sheline 2000 post-traumatic stress disorder Kasai et al 2008 are associated with decreased density or volume of the hippocampus. If an object were extremely dense it would feel heavier than another object of the same size that was less dense. The density of water increases with decreasing temperature reaching a maximum at 40 C 40 C and then decreases as the temperature falls below 40 C 40 C.
Just like a solid the density of a liquid equals the mass of the liquid divided by its volume. The density ρ of an object is defined as the ratio of its mass to its volume. Density can be useful in identifying substances.
GalThis is equal to a rounded value of 1 gram per milliliter gml or 1 gram per cubic centimeter gcm 3 or 1000 kgm 3. Students measure the volume and mass of water to determine its density. The density more precisely the volumetric mass density.
22 A to 22 C. The density of most of the substances decreases with the increase in temperature and increases with decrease in temperature. It is measured throughout industry to gain insight into materials for example their purity concentration of components and composition.
ν or is an intrinsic property of the substance defined as the ratio of the substances volume V to its mass mIt is the reciprocal of density and it is related to the molar volume and molar mass. The density of water at 4 degrees Celsius is 8345 lbsUS. Relative density of a substance is defined as the ratio between the density of the.
Table 142 shows the density of water in various phases and temperature. Learners do not need to do calculations at this level but if you would like to extend the exercise you could work out the densities of each liquid using the. Water has a maximum density at 392ºF or 4ºC.
The density of water is roughly 1 gram per milliliter but this changes with temperature or if there are substances dissolved in it. Heating a substance causes molecules to speed up and spread slightly further apart occupying a larger volume that results in a decrease in densityHot water is less dense and will float on room-temperature water. Also known as specific mass of a substance is its mass per unit volumeThe symbol most often used for density is ρ the lower case Greek letter rho although the Latin letter D can also be used.
For example a 1-litre soda bottle filled with air would feel much lighter than the same bottle filled with water. Adding additional matter to the same volume also increases density even if the matter added is a different type of matter Fig. Where ρ is the density m is the mass and V is the volume.
Free online Density calculator with which you can calculate the density of any substance given its mass and volume. The density of a substance is the same regardless of the size of the sample. Thus the density of water is maximum at 40C.
22 A to 22 D.

Dilution Of Solutions Calculator Chemistry Solutions Calculator

The Mass Per Unit Volume Of A Substance Is Called Density Density Mass Volume Arrange The Following In Order Of Increasing Density Air Exhaust From Chimneys Honey Water Chalk Cotton And
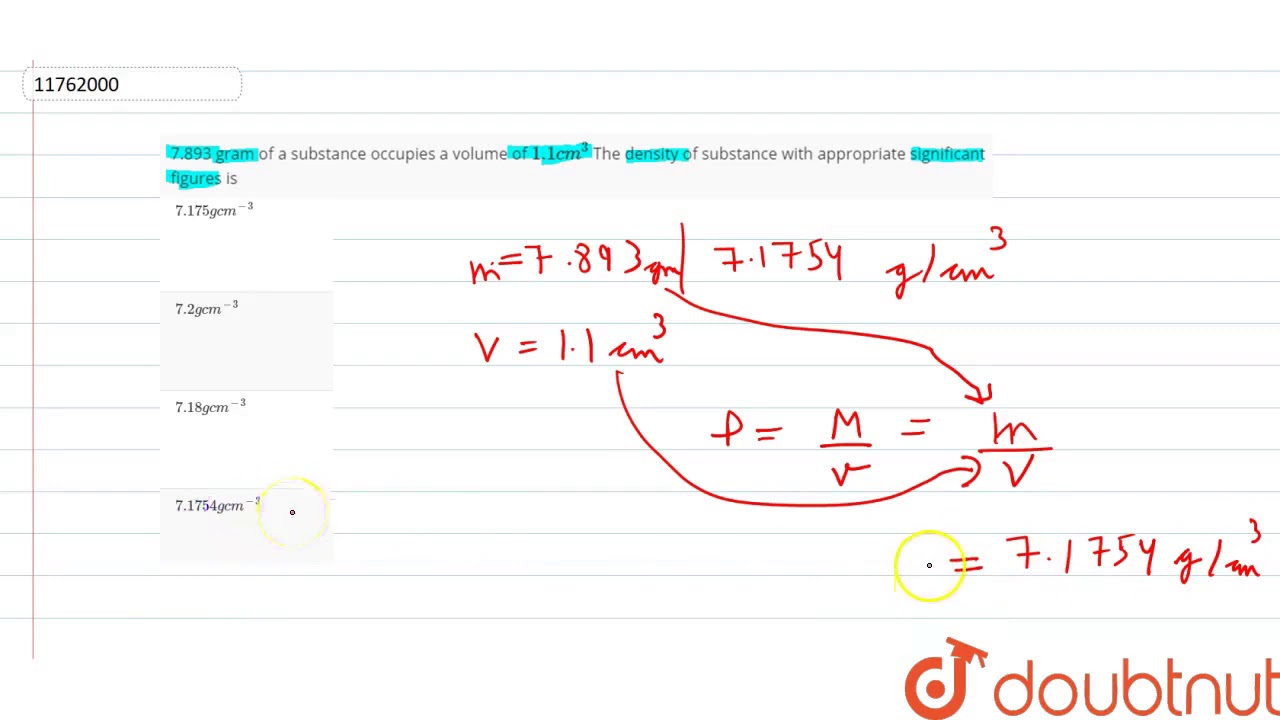
As The Density Of A Substance Increases What Happens To The Volume Lisbdnet Com
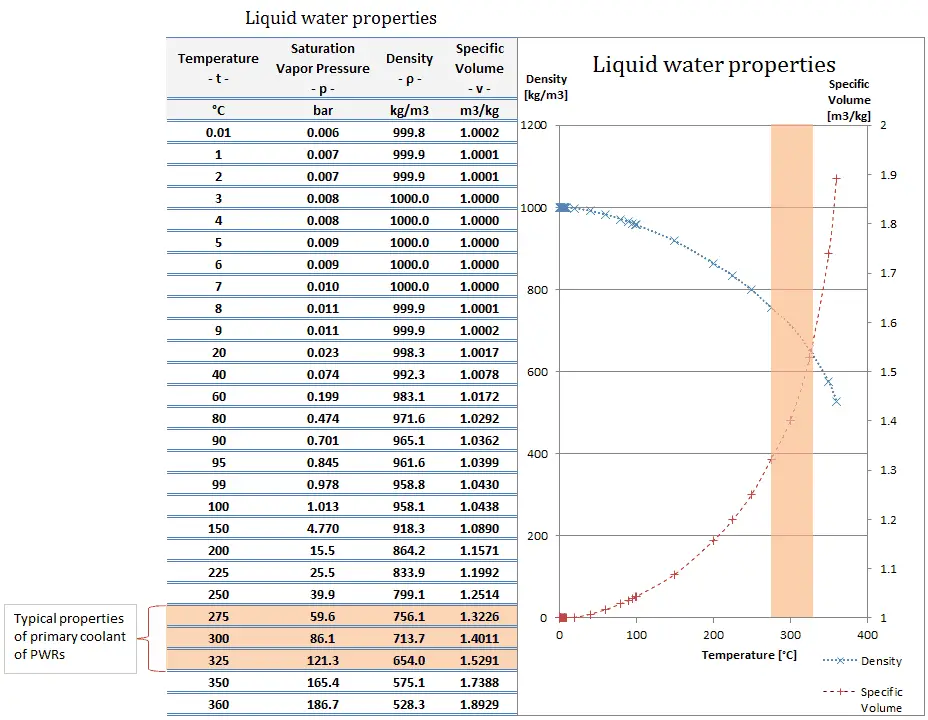
What Is Density Of Water Specific Volume Of Water Definition

The Mass Per Unit Volume Of A Substance Is Called Density Density Mass Volume Arrange The Following In Order Of Increasing Density Air Exhaust From Chimneys Honey Water Chalk Cotton And

What Is Density Chapter 3 Density Middle School Chemistry

Volume And Density Introduction To Chemistry

As The Density Of A Substance Increases What Happens To The Volume Lisbdnet Com
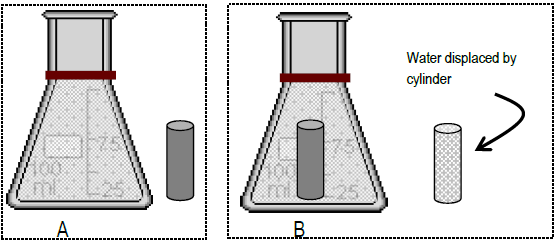
Lab 1 Density Determinations And Various Methods To Measure Volume
:max_bytes(150000):strip_icc()/digital-illustration-of-showing-shape-and-volume-of-solid-gas--and-liquid-gas-taking-shape-of-conica-91284996-5ba786d2c9e77c0082827f47.jpg)
Specific Volume Definition Formulas Examples
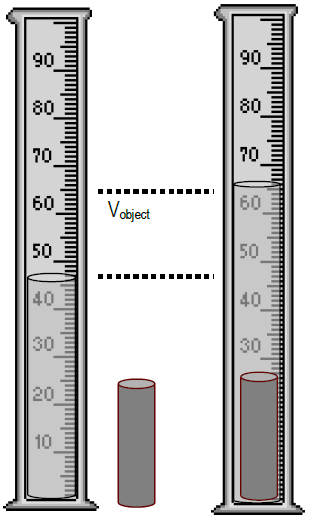
Lab 1 Density Determinations And Various Methods To Measure Volume

Temperature And Density Chapter 3 Density Middle School Chemistry

Q37 How Does The Density Of A Lido

Ideal Gas Law The Equation Of State Of A Hypothetical Ideal Gas It Is A Good Approximation To The Behavior Of Ideal Gas Law Chemistry Education Gas Constant

Pin On Best Chemistry Calculator

Chris Christensen Spectrum One Coarse And Rough Coat Substance Builder Substances Rough Spectrum

Cie Igcse Chemistry Complete Notes Istruzione

Chris Christensen Spectrum One Coarse And Rough Coat Substance Builder In 2022 Shampoo Substances Rough

Comments
Post a Comment